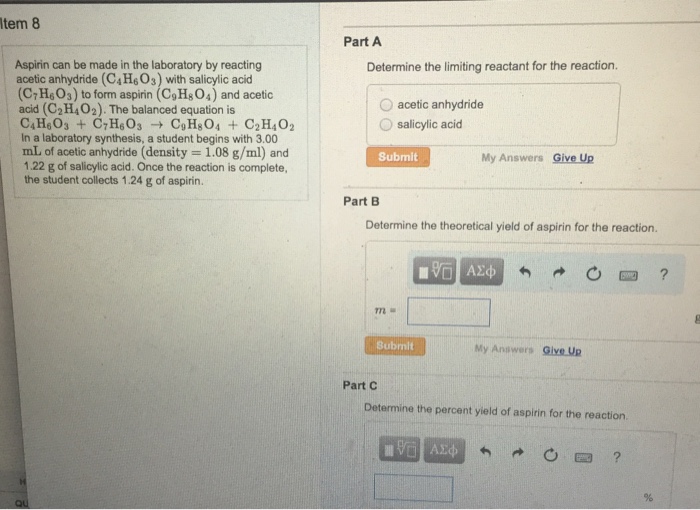
Solved Aspirin can be made in the laboratory by reacting
Being a carboxylic acid anhydride, acetic anhydride will slowly decay in contact with water into acetic acid. This process, however, is slow enough for aqueous solutions to be made and used immediately. Physical. Acetic anhydride is a colorless liquid with a density slightly greater than that of water.

acetic anhydride vapor density
Acetic anhydride Formula: C 4 H 6 O 3 Molecular weight: 102.0886 IUPAC Standard InChI: InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey: WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: 108-24-7 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file

acetic anhydride chemical formula
Ethanoic anhydride is a colorless liquid, smelling strongly of vinegar (ethanoic acid). The smell is because ethanoic anhydride reacts with water vapor in the air (and moisture in your nose) to produce ethanoic acid again. This reaction with water is given in detail on another page. (Find it from the acid anhydrides menu - link at the bottom of.

Preparation of Acetic Anhydride Acetic anhydride, Chemistry notes, Sodium acetate
acetic anhydride - cas 108-24-7, synthesis, structure, density, melting point, boiling point

Acetic Anhydride Density, Formula & Uses Video & Lesson Transcript
Catalog Number 822278 Product Name Acetic anhydride Select Language If you are not able to see the SDS please use this link to download it: Download SDS View or download the Acetic anhydride MSDS (Material Safety Data Sheet) or SDS for 822278 from Merck.

Acetic Anhydride 500ml
Description Acetic anhydride appears as a clear colorless liquid with a strong odor of vinegar. Flash point 129 °F. Corrosive to metals and tissue. Density 9.0 lb /gal. Used to make fibers, plastics, pharmaceuticals, dyes, and explosives. CAMEO Chemicals Acetic anhydride is an acyclic carboxylic anhydride derived from acetic acid.

1. What precautions should you take when working
Acetic anhydride. Molecular Formula C13CHO. Average mass 104.074 Da. Monoisotopic mass 104.038406 Da. ChemSpider ID 17341077. - Non-standard isotope.

Solved How many moles are in 8 5 mL of acetic anhydride
Acetic anhydride Formula: C 4 H 6 O 3 Molecular weight: 102.0886 IUPAC Standard InChI: InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey: WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: 108-24-7 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file
[Solved] The density of acetic anhydride is 1.08g/ml calculate the mass of... Course Hero
20.18: Reactions of Anhydrides. Page ID. T his page explains what acid anhydrides are and looks at their simple physical properties such as boiling points. It introduces their chemical reactivity in a general way. A carboxylic acid such as ethanoic acid has the structure: If you took two ethanoic acid molecules and removed a molecule of water.

Solved (a) How many moles of acetic anhydride were replaced
Vapor Density: 3.5 (air=1) Evaporation Rate:0.46 (n-butyl acetate=1) Viscosity: 0.91mPa.s @ 20 deg C Boiling Point: 140 deg C @ 760mmHg. Since acetic anhydride is a relatively non-volatile liquid, direct venting of the vapor to the atmosphere from a hole in a ruptured vessel does not consitiute a significant hazard downwind. Only vapor.

Solved CEIIIL PrelabAspirin Name 1. Acetic anhydride is a
The molar mass of acetic anhydride is 102.1 g/mol and its density is 1.080 g/mL? Solution The formula for density is ¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯¯∣∣ a a ρ = m V a a ∣∣ −−−−−−−−−−−− where ρ is the density, m is the mass, and V is the volume of the sample. We can rearrange the formula to get m = V ×ρ mass = 10.00mL × 1.080 g 1mL = 10.80 g
acetic anhydride vapor density
Acetic anhydride Write a review 99.5% Synonym (s): Acetanhydride, Acetic acid anhydride, Acetyl acetate, Acetyl anhydride, Ethanoic anhydride Linear Formula: (CH3CO)2O CAS Number: 108-24-7 Molecular Weight: 102.09 Beilstein: 385737 EC Number: 203-564-8 MDL number: MFCD00008705 PubChem Substance ID: 24878429 NACRES: NA.21

Acetyl Chloride Uses Chemistry notes, Acetic anhydride, Chemistry
IUPAC identifier Office of Data and Informatics IUPAC Standard InChI:InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey:WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: Chemical structure: This structure is also available as a 2d Mol file 3d SD file The 3d structure may be viewed using

Solved 1. Aspirin can be made in a laboratory by reacting
This monograph for Acetic Anhydride provides, in addition to common physical constants, a general description including typical appearance, applications, change in state (approximate), aqueous solubility, and density. The monograph also details the following specifications and corresponding tests for verifying that a substance meets ACS Reagent Grade specifications including: Assay, Residue.
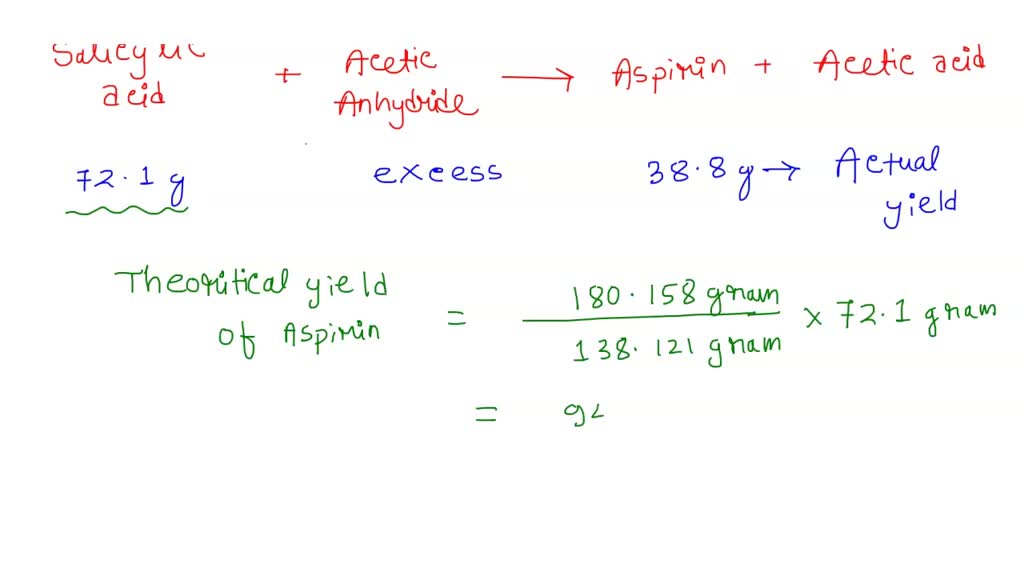
SOLVED Aspiring (CoHgO4) is made by reacting salicylic acid (C,HsO3) with acetic anhydride
Acetic anhydride is an organic compound that is composed of carbons, hydrogens, and oxygens. This organic molecule is the simplest carboxylic acid anhydride, which is a functional group that.
[Solved] Appearance of acetic anhydride Volume of acetic anhydride Density... Course Hero
Acetic anhydride Formula: C 4 H 6 O 3 Molecular weight: 102.0886 IUPAC Standard InChI: InChI=1S/C4H6O3/c1-3 (5)7-4 (2)6/h1-2H3 IUPAC Standard InChIKey: WFDIJRYMOXRFFG-UHFFFAOYSA-N CAS Registry Number: 108-24-7 Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file